Abstract
Introduction
Central nervous system (CNS) relapse (CNSr) in patients with aggressive non-Hodgkin lymphoma (NHL) occurs uncommonly (estimated incidence 5%) but carries a high morbidity and mortality. Studies have identified risk factors for CNSr such as high tumor burden and extranodal (EN) disease. However, most focus on B-cell NHL with minimal data in T-cell lymphomas (TCL), which are significantly less common and more heterogenous. The few small series of CNSr in TCL report a median overall survival (OS) less than 3 months (mo) with incidence ranging from 2.6-9%. To better define CNSr in TCL, we performed a multi-institutional retrospective analysis of TCL patients with CNSr and herein describe clinicopathologic characteristics and treatment of CNSr.
Methods
We performed a retrospective observational study using data from 9 US academic centers with IRB approval at individual sites. We included adult patients diagnosed with a mature T-cell neoplasm as per the 2016 WHO classification between 1/1/2009-1/1/2019, who were found to have CNSr at any time after initial diagnosis, and collected patient, disease, and treatment characteristics at time of initial diagnosis as well as at CNSr. Patients with a diagnosis of a precursor T-cell malignancy or with CNS disease identified at initial TCL diagnosis (TCLd) and/or prior to first-line systemic treatment were excluded.
Results
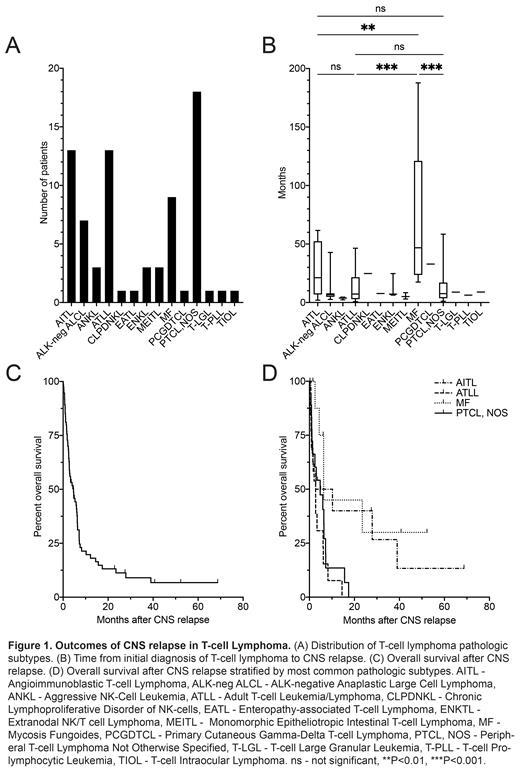
In this analysis, we report the outcomes of 75 patients (male n=45, female n=30). At TCLd, the median age was 59 years (range 20-81), and 61% of patients (n=46) had an IPI score of at least 3, 92% (n=69) had EN involvement with 37% (n=28) involving at least 2 EN sites, and 59% (n=44) had BM involvement. The most common pathologic diagnoses were peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS; 24%, n=18), angioimmunoblastic T-cell lymphoma (AITL; 17%, n=13), adult T-cell leukemia/lymphoma (ATLL; 17%, n=13), and mycosis fungoides (MF; 12%, n=9) (Figure 1A). First-line systemic therapy for TCL included anthracyclines for 72% (n=54). Autologous and allogenic transplants were performed prior to CNSr in 12% (n=9) and 8% (n=6) of patients, respectively. Prior to CNSr, 48% (n=36) had non-CNS relapse. Some form of CNS prophylaxis was used during initial systemic lymphoma therapy in 24% of patients (n=18), predominantly intrathecal methotrexate (IT MTX; n=16).
Median time from TCLd to CNSr was 8.5mo, though this was significantly longer in MF (46.8mo [range 17.5-187.7]) versus PTCL, NOS (7.6mo [range 1.1-58.4], P=0.0002), AITL (21.2mo [range 2.0-61.6, P=0.008), and ATLL (7.3mo [range 0.7-46.4], P=0.0005) (Figure 1B). CNSr developed within 6mo of TCLd in 31% of patients (n=23) and within 12mo in 57% (n=43). Symptoms related to CNSr occurred in 71% of patients (n=53). CNSr patterns were 61% leptomeningeal (n=46), 21% parenchymal (n=16), and 17% both (n=13) with no significant survival difference between leptomeningeal or parenchymal disease alone (HR 1.45, 95% CI 0.78-2.70, P=0.28). Concomitant systemic relapse was observed in 59% of patients (n=44). The most common CNS-directed therapy for CNSr was IT MTX (56%; n=42), though multiple different IT and/or systemic regimens were used. Patients received a median of 1 line of CNS-directed treatment (range 0-5). The overall response rate to initial CNS directed treatment was 32% (16% CR, 16% PR). Median follow up after CNSr was 40.7mo. At last follow up, 83% had died (n=62). Median OS after CNSr was 4.6mo (range 0.1-68.7) (Figure 1C). Those with ATLL had the shortest median OS after CNSr (2.7mo) versus 6.3mo in MF (HR 3.69, 95% CI 1.44-9.42, P=0.005), 6.5mo in AITL (HR 2.16, 95% CI 0.92-5.06, P=0.054), and 4.8mo in PTCL, NOS (HR 1.49, 95% CI 0.70-3.19, P=0.27) (Figure 1D). The most common cause of death was progressive lymphoma (77%; n=48).
Conclusions
This is to our knowledge the largest series of CNSr in TCL to date. Most CNSr occurred within 12mo, though CNSr occurred later in patients with MF. Although the prognosis after CNSr was generally poor, we found that median OS in CNSr was longer than previously reported, perhaps reflecting more effective treatments for CNS and systemic relapse, inclusion of MF, or lead time bias. Further analysis of the impact of different treatment strategies and outcomes in CNSr was limited by the small sample size and heterogeneity within our cohort, and future analyses in a larger cohort should focus on factors associated with outcomes.
Horwitz: ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio.: Consultancy, Research Funding; Affimed: Research Funding; Aileron: Research Funding; Acrotech Biopharma, Affimed, ADC Therapeutics, Astex, Merck, Portola Pharma, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Th: Consultancy; Celgene: Research Funding; C4 Therapeutics: Consultancy; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kura Oncology: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Research Funding; Secura Bio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Verastem/Securabio: Research Funding. Bennani: Purdue Pharma: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Kyowa Kirin: Other: Advisory Board; Vividion: Other: Advisory Board; Kymera: Other: Advisory Board; Verastem: Other: Advisory Board. Chavez: AstraZeneca: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Merk: Research Funding; MorphoSys, AstraZeneca, BeiGene, Genentech, Kite, a Gilead Company, and Epizyme: Speakers Bureau; MorphoSys, Bayer, Karyopharm, Kite, a Gilead Company, Novartis, Janssen, AbbVie, TeneoBio, and Pfizer: Consultancy; BMS: Speakers Bureau. Sokol: Dren Bio: Membership on an entity's Board of Directors or advisory committees; Kyowa-Kirin: Membership on an entity's Board of Directors or advisory committees. Saeed: Nektar Therapeutics: Consultancy, Other: research investigator; MEI Pharma Inc: Consultancy, Other: investigator; Celgene Corporation: Consultancy, Other: investigator; MorphoSys AG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb Company: Consultancy; sano-aventis U.S.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutica Products, LP: Consultancy, Other: investigator; Kite Pharma: Consultancy, Other: investigator; Other-TG therapeutics: Consultancy, Other: investigator; Other-Epizyme, Inc.: Consultancy; Other-Secura Bio, Inc.: Consultancy; Seattle Genetics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mehta-Shah: Kiowa Hakko Kirin: Consultancy; C4 Therapeutics: Consultancy; Verastem: Research Funding; Karyopharm: Consultancy; Ono Pharmaceuticals: Consultancy; Secura Bio: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; AstraZeneca: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding; Innate Pharmaceuticals: Research Funding; Roche/Genentech: Research Funding; Corvus Pharmaceuticals: Research Funding. Olszewski: TG Therapeutics: Research Funding; PrecisionBio: Research Funding; Celldex Therapeutics: Research Funding; Acrotech Pharma: Research Funding; Genentech, Inc.: Research Funding; Genmab: Research Funding. Allen: Epizyme: Consultancy; MorphoSys: Consultancy; ADC Therapeutics: Consultancy; Secure Bio: Consultancy; Kyowa Kirin: Consultancy. Gerson: Abbvie: Consultancy; Kite: Consultancy; TG Therapeutics: Consultancy; Pharmacyclics: Consultancy. Landsburg: Triphase: Research Funding; Takeda: Research Funding; Curis: Research Funding; ADCT: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: DSMB member; Incyte: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees. Schuster: Adaptive Biotechnologies: Research Funding; Pharmacyclics: Research Funding; Merck: Research Funding; Genentech/Roche: Consultancy, Research Funding; Tessa Theraputics: Consultancy; Juno Theraputics: Consultancy, Research Funding; Loxo Oncology: Consultancy; BeiGene: Consultancy; Alimera Sciences: Consultancy; Acerta Pharma/AstraZeneca: Consultancy; Abbvie: Consultancy, Research Funding; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Incyte: Research Funding; TG Theraputics: Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Svoboda: Atara: Consultancy; Adaptive: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Imbrium: Consultancy; Pharmacyclics: Consultancy, Research Funding; Genmab: Consultancy; Merck: Research Funding; Incyte: Research Funding; BMS: Consultancy, Research Funding; TG: Research Funding; Seattle Genetics: Consultancy, Research Funding. Barta: Kyowa Kirin: Honoraria; Acrotech: Honoraria; Daiichi Sankyo: Honoraria; Seagen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal